GOG Foundation
GOG Foundation Overview
The GOG Foundation has one mission that is dedicated to transforming
the standard of care in gynecologic oncology.
350+ Clinical Trials
400+ Participating Sites
115000+ Patients
Groundbreaking research, sincere impact.
The GOG Foundation, Inc. (GOG Foundation) is a nonprofit organization with the purpose of promoting excellence in the quality and integrity of clinical and basic scientific research in the field of gynecologic malignancies, including cancers that arise from the ovaries, uterus, cervix, vagina, and vulva. Our institutions and investigators are essential contributors to advancements in treatment regimens, surgical procedures, quality of life analyses, and prevention knowledge. The results of GOG Foundation clinical trials have influenced the standard of care for numerous malignant gynecologic neoplasms.
Our Mission
The mission of the GOG Foundation is to conduct clinical and translational research that positively impacts patients through the prevention and treatment of gynecologic malignancies.
Our Vision
The vision of the GOG Foundation is to be the premier collaborative network for transformative research in gynecologic malignancies.
Critical to the mission
The GOG Continuing Medical Education (CME) Program is dedicated to enhancing the knowledge of physicians and other healthcare professionals focused on improving the lives of cancer patients.
The GOG Foundation disseminates the clinical implications of research findings to its participating investigators and other health-care professionals around the world, as well as to cancer survivors, their families, support groups, and advocates.

Dr. Alvarez speaking in Philadelphia
Research Structure
The GOG Foundation is the home of GOG Partners and the NRG Oncology gynecologic program.
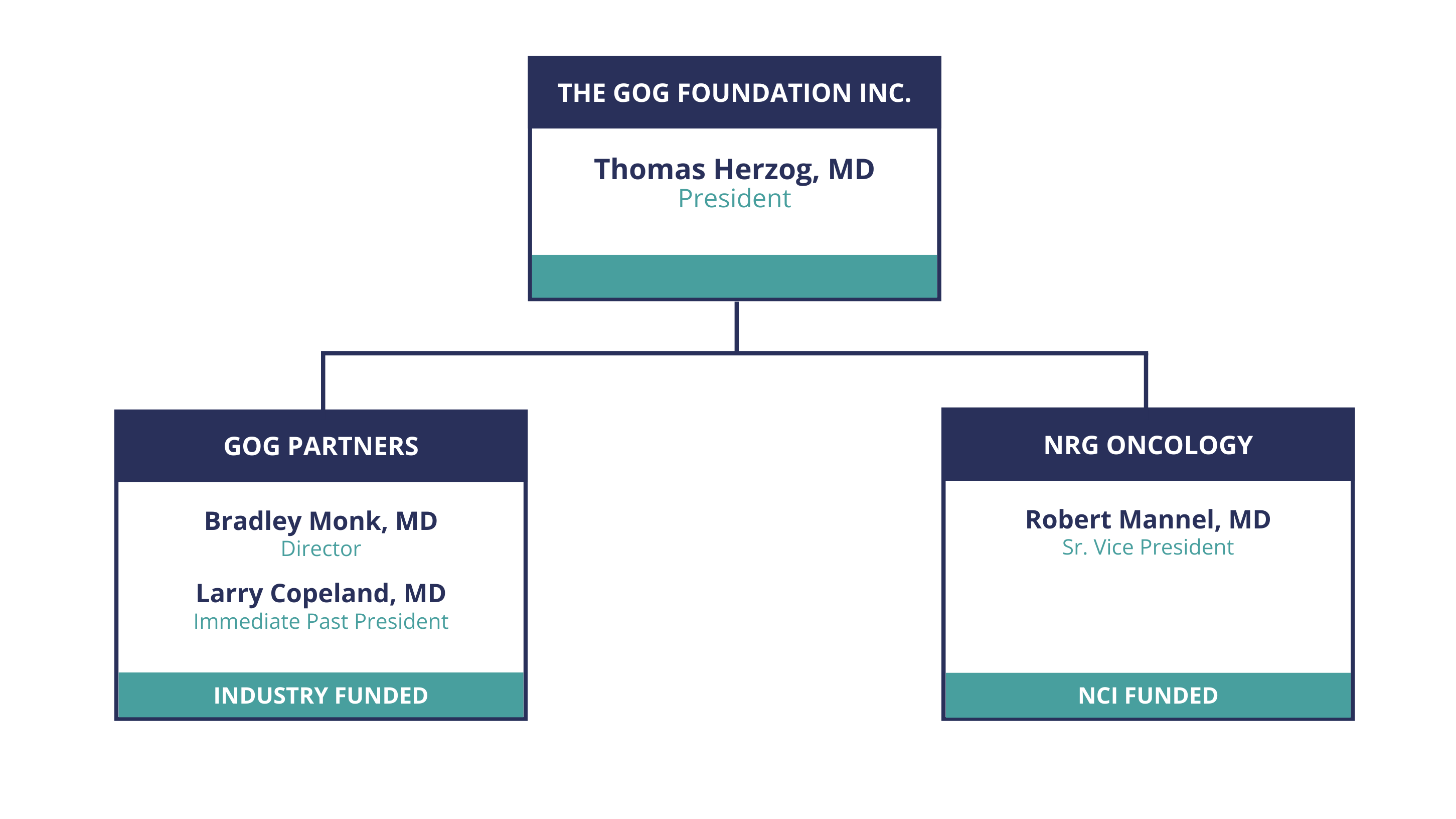

Supported by industry, GOG Partners has been structured to work directly with pharmaceutical organizations and operate clinical trials outside the National Cancer Institute (NCI) framework. By providing an alternative venue for patient accrual and site infrastructure support, GOG Partners has helped stabilize the national gynecologic clinical trials network.

The federally-funded NRG Oncology conducts gynecologic cancer trials sponsored by the NCI. The 501(c)(3) was formed by the Gynecologic Oncology Group (a predecessor to the GOG Foundation), the National Surgical Adjuvant Breast and Bowel Project, and the Radiation Therapy Oncology Group.
Leadership

Dr. Thomas Herzog
President
Dr. Thomas J. Herzog took office as The GOG Foundation, Inc. President on July 20, 2023. Dr. Herzog brings a comprehensive background in clinical trials, the integral business aspects and acumen to this important position. A practicing gynecologic oncologist and member of the Board of Directors of GOG-F, he has served as the Treasurer of GOG-F from 2014-2023 and prior to taking presidential office, served as Associate Director of the GOG Partners (GOG-P) program. He is currently Deputy Director of the University of Cincinnati Cancer Center and Paul & Carolyn Flory Professor in the department of Obstetrics and Gynecology at the University of Cincinnati.

Dr. Robert S. Mannel
Senior Vice President
Dr. Robert Mannel serves as Sr. Vice President of the GOG Foundation, as well as NRG Oncology Group Chair. He is the Rainbolt Family Endowed Chair in Cancer and Professor of Gynecologic Oncology in the Department of Obstetrics and Gynecology at the University of Oklahoma Health Sciences Center (OUHSC).
Executive Leadership
GOG Partners

Dr. Bradley Monk
Director

Dr. Kathleen Moore
Associate Director

Dr. Larry J. Copeland
Immediate Past President

Dr. Robert Coleman
Special Advisor to the President
NRG Oncology

Dr. Carol Aghajanian
Gynecologic Cancer Committee Chair

Dr. Paul DiSilvestro
Gynecologic Cancer Committee Vice Chair
Ovarian Cancer Leadership
GOG Partners

Dr. David O'Malley
Ovarian Trial Advisor
NRG Oncology

Dr. Kathleen Moore
Ovarian Committee Chair

Dr. Joyce Liu
Ovarian Committee Vice Chair
Uterine Cancer Leadership
GOG Partners

Dr. Brian Slomovitz
Uterine Trial Advisor
NRG Oncology

Dr. Matthew Powell
Uterine Cancer Committee Chair

Dr. Ann Klopp
Uterine Cancer Committee Vice Chair
Cervix Cancer Leadership
GOG Partners

Dr. Leslie Randall
GOG Partners - Cervix Trial Advisor
NRG Oncology

Dr. Charles Leath
Cervix/Vulva Cancer Committee Chair

Dr. Jyoti Mayadev
Cervix/Vulva Cancer Committee Vice Chair

Dr. Dmitriy Zamarin
Cervix/Vulva Cancer Committee Vice Chair
Rare Tumor Leadership
NRG Oncology

Dr. Allan Covens
Rare Tumor Committee Chair

Dr. Jubilee Brown
Rare Tumor Committee Vice Chair
Developmental Therapeutics Leadership
NRG Oncology

Dr. Roisin O'Cearbhaill
Developmental Therapeutics Committee Chair

Dr. Floor Backes
Developmental Therapeutics Committee Vice Chair
Associate Clinical Trial Advisors
GOG Partners

Dr. Ramez Eskander

Dr. Bhavana Pothuri
Administrative Executives
Katie Campbell
Executive Director, GOG Partners, Clinical Trials Management
Jenna Cummins
Executive Director of Business Development
Michael Powers
General Counsel, The GOG Foundation, Inc.
Laura L. Reese
Executive Director, Operations
Mary C. Sharp
Chief Financial Officer
Committees
- GOG Partners Leadership Committee
- GOG Partners Publications Committee
- Quality and Assessment (QAAC/Membership) Committee
- NRG Oncology GYN Cancer Committee (Disease Site Chairs/Co-Chairs)
- Communications Committee
- Development Committee
- Education and Mentoring Committee
- International Relations Committee
History
Over the course of 50 years, the GOG Foundation has evolved to become a leader among clinical trial groups studying gynecologic cancer.
The Gynecologic Oncology Group, the earliest version of today’s GOG Foundation, was founded in 1970 and housed within the American College of Obstetricians Gynecologists. It later became an independent nonprofit with the formation of a 501(c)(3), providing a corporate home for NCI-sponsored cooperative group clinical trials. Since then, the GOG developed into a large organization with a robust trial portfolio that enjoyed extensive support from the gynecologic cancer research community.
Starting with the present
“We are going to have a great new generation of researchers in GYN oncology that are instilled with a passion and commitment to their field and their patients. From that passion is where you are going to see the great discoveries.”
2019
GOG-258 shows adjuvant chemo-radiation does not improve relapse free survival vs chemotherapy alone in women with stage III or IV endometrial cancer.
2019
GOG-213 shows secondary cytoreduction did not improve overall survival in patients with recurrent platinum sensitive ovarian cancer treated with chemotherapy and bevacizumab.
2017
Dr. Larry J. Copeland is elected President of the GOG Foundation.
2014
GOG-240 shows improved survival when bevacizumab is added to chemotherapy for advanced cervical cancer.
2014
The Gynecologic Oncology Group (GOG) is restructured into The GOG Foundation, Inc.
2013
The GOG, in partnership with the National Surgical Adjuvant Breast and Bowel Project and the Radiation Therapy Oncology Group, forms a new 501(C)(3) after the National Clinical Trials Network is reorganized. This new entity, NRG Oncology Foundation, Inc., becomes the corporate home for NCI-funded trials.
2012
GOG-0173 demonstrates that sentinel node biopsy is effective in select women with vulvar cancer.
2011
GOG-0218 improves PFS when bevacizumab was added to frontline chemotherapy and continued as maintenance in patients with stage III/IV ovarian cancer.
2010
The GOG grows to nearly 200 member institutions across the country.
2006
GOG-0172 intraperitoneal chemotherapy improved PFS and OS in women with stage III optimally debulked ovarian cancer.
2003
The Gynecologic Oncology Group is incorporated.
“The GOG has fundamentally set the standards for cervical cancer, endometrial cancer, ovarian cancer management, but it also is looking forward to where we want to be.”
2002
Dr. Philip J. DiSaia elected as GOG Group Chair.
2000
By the turn of the century, the GOG had grown within the Cancer Therapy Evaluation Program (CTEP) and NCI portfolio to become a significant contributor in defining the standard of care in gynecologic oncology.
1999
NCI issues alert that chemo-radiation should be considered for all patients with cervical cancer based on findings from GOG-85 (collaboration with SWOG) and four other randomized trials.
1996
GOG-111 is a landmark study that led to incorporation of taxanes into frontline chemotherapy for advanced ovarian cancer.
1993
The GOG Quality of Life Committee was formed and Protocol 9102 was first protocol with a primary endpoint of patient-reported effects of chemotherapy, paving the way for GOG’s continued dedication to improving the quality of life for our patients.
1992
Hoskins et al publish The Influence of Cytoreductive Surgery on recurrence-free interval and Survival in small volume Stage III Epithelial Ovarian Cancer based on secondary analysis of GOG Protocol 52.
1988
Dr. Robert Park is elected Group Chairman.
1987
GOG-33 leads to surgical staging of endometrial cancer.
1976
The first clinical publication of the GOG, the preliminary results of Pilot 1, surgical staging of endometrial cancer. By the end of the decade, a total of 40 manuscripts would be published.
1975
The GOG grows to 34 institutions, covering about 4,000 new invasive cancers a year, with 23 protocols activated.
1970
The Gynecologic Oncology Group (GOG) is founded and housed within the American College of Obstetricians and Gynecologists.
For a more comprehensive review of GOG's history and research, download our book.
Download